Abstract
Background: Despite changes in treatment regimens, the patient's age (and the corresponding treatment intensity) remains one of the most important prognostic factors in AML. Thus far, only 5-15% of AML patients aged ≥60 years (y) are cured with chemotherapy. Identification of patients who are more (or less) likely to respond to standard cytarabine/daunorubicin-based chemotherapy might help to enable early risk stratification toward alternative treatment regimens.
Aims: To provide a comprehensive molecular profile of a large cohort of adults with de novo acute myeloid leukemia (AML) aged ≥60 y, and to determine if specific molecular and/or cytogenetic features could identify patients who are more likely to respond to standard chemotherapy.
Methods: We used a next-generation sequencing panel of 80 cancer- and/or leukemia-associated genes to profile molecularly 423 patients aged ≥60 y with de novo AML and available cytogenetic data similarly treated on Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology.
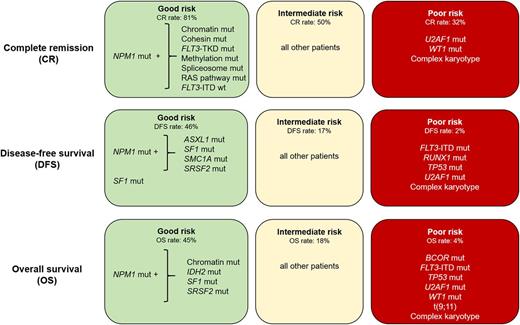
Results: Overall, we detected 1377 mutations, with a median of 3 mutations per patient (range: 0-8). The outcome of the whole patient cohort was poor, with complete remission (CR) rate of 55% and disease-free (DFS) and overall survival (OS) 3y-rates of 14%. In multivariable analyses (MVA), mutated NPM1 was the only factor positively associated with achieving CR (P <.001). The presence of U2AF1 mutations (P=.03), WT1 mutations (P=.01), or a cytogenetically normal karyotype (CN-AML; P=.03) were associated with lower CR rates. We also found that CR rates of NPM1 -mutated patients varied from 50 to 95% depending on specific co-mutations. Patients who in addition to a NPM1 mutation harbored mutations in cohesin complex (SMC1A, SMC3 or STAG2) or chromatin remodeling (ASXL1, BCOR, BCORL1, EZH2 or SMARCA2) genes had CR rates of 95% and 89%, respectively, and those with simultaneous FLT3 tyrosine kinase domain mutations (FLT3 -TKD) or mutations in the methylation-related (DNMT3A, IDH1 / 2 or TET2) or RAS (KRAS, NRAS, or PTPN11) genes had CR rates of ~83%, compared with only 40% in patients without these coexisting mutations (P <.006). In MVA for DFS, the presence of NPM1 (P <.001) and SF1 mutations (P=.04) associated with longer DFS, with 22% of NPM1 -mutated patients and 40% of SF1 -mutated patients being disease-free 3 y after diagnosis, compared with only 8% of patients without these mutations. The presence of FLT3 internal tandem duplication (FLT3 -ITD; P <.001), and complex karyotype (CK) (P <.001) were associated with poor DFS. In MVA for OS, NPM1 mutations associated with improved OS (P <.001). The presence of FLT3 -ITD (P <.001), BCOR mutations (P=.003), TP53 mutations (P=.003), t(9;11) (P=.03) or CK (P=.002) was associated with shorter OS. Using the variables identified in our multivariable models, refined by the consideration of the presence or absence of co-occurring mutations in NPM1 -mutated AML, we classified the patients into good, intermediate and poor risk for CR attainment, DFS and OS (Figure 1). Whereas 81% (103/127) of good-risk patients (comprising NPM1 -mutated patients harboring mutations in chromatin remodeling or cohesin complex genes, FLT3 -TKD, methylation-related genes, spliceosome genes, RAS pathway, and/or patients without FLT3 -ITD) achieved a CR,only 32% (30/95) of poor-risk patients did (comprising patients with U2AF1 mutations, WT1 mutations and/or CK). Intermediate-risk patients had a 50% (100/201) CR rate. Similarly, using NPM1 co-mutation patterns and SF1 mutation status, we identified patients with favorable DFS and OS 3-year rates of 46% and 45% (representing 8% and 10% of all patients), respectively. In contrast, patients with adverse molecular features [DFS: the presence of FLT3 -ITD, RUNX1, TP53 or U2AF1 mutations and/or typical or atypical CK; OS: the presence of BCOR mutations, FLT3 -ITD, TP53, U2AF1 or WT1 mutations, t(9;11), and/or typical CK] had DFS and OS rates of only 2% and 4%.
Summary: We identified combinations of mutations whose presence is associated with favorable outcome in older AML patients treated with standard chemotherapy. Patients with intermediate and adverse molecular features should be considered for alternative treatment regimens.
Stone: Otsuka: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Ono: Membership on an entity's Board of Directors or advisory committees; Orsenix: Membership on an entity's Board of Directors or advisory committees; Cornerstone: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Argenix: Other: DSMB; Arog: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Actinium: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Fujifilm: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Jazz: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Seattle genetics: Membership on an entity's Board of Directors or advisory committees; Sumitomo: Membership on an entity's Board of Directors or advisory committees. Kolitz: Gilead, Magellan, Novartis, Pharmacyclics, and Seattle Genetics: Consultancy; Gilead, Magellan, and Novartis: Honoraria; Celgene, Jazz: Equity Ownership; Boehringer Ingelheim, Cantex, Erytech, and Millennium: Research Funding; Gilead, Novartis, and Seattle Genetics: Other: Travel Support. Powell: Rafael Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Byrd: Janssen: Research Funding; Genentech: Research Funding; Acerta Pharma: Research Funding; Pharmacyclics: Research Funding; The Ohio State University: Patents & Royalties: OSU-2S.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal